Explainer: Cognitive Load Theory
Humans can only process a limited amount of information in a given length of time. This is the central phenomenon which Cognitive Load responds to. It will be familiar to you! Forgetting someone’s name within half an hour of being introduced to them, taking down a phone number in three-digit bursts rather than all at once, writing a shopping list rather than holding it in your head. These are all related to the speed at which we process information.
Formal work in psychology has theorised this phenomenon by appeal to the concept of ‘working memory’. While our long-term memory seems to be more-or-less limitless, dealing with immediate-and-new information takes up a limited amount of ‘mental space’. Processing and assimilating new information is a process with a radically-limited rate.
One Model of Cognitive Load
There are several models of cognitive load. I am limiting the discussion here to the core features of the one I have found most useful: Sweller’s. This has four main principles, in my understanding.
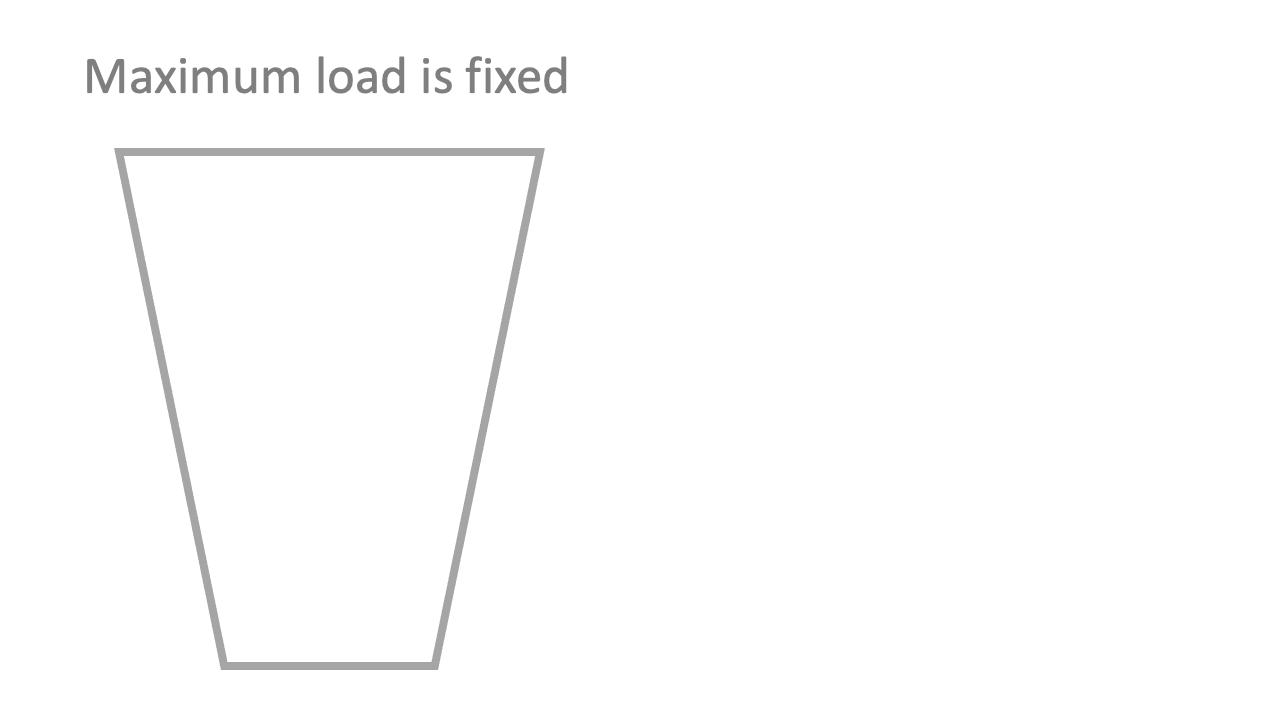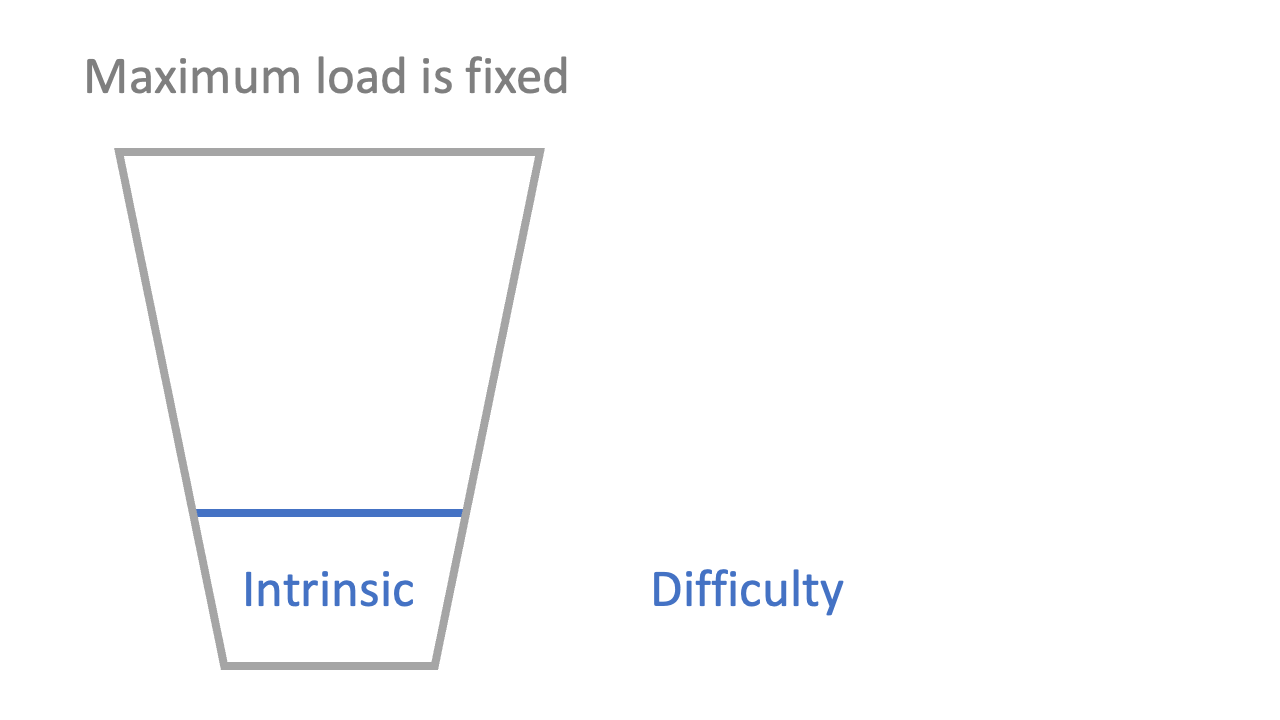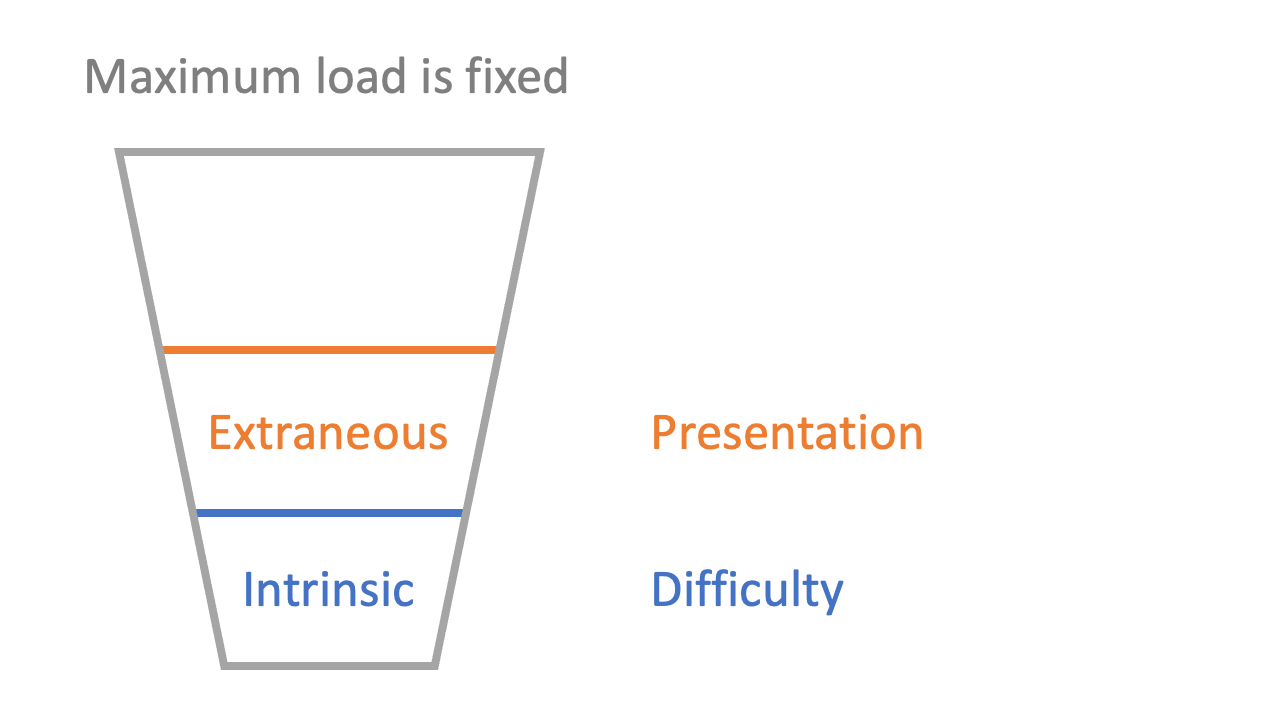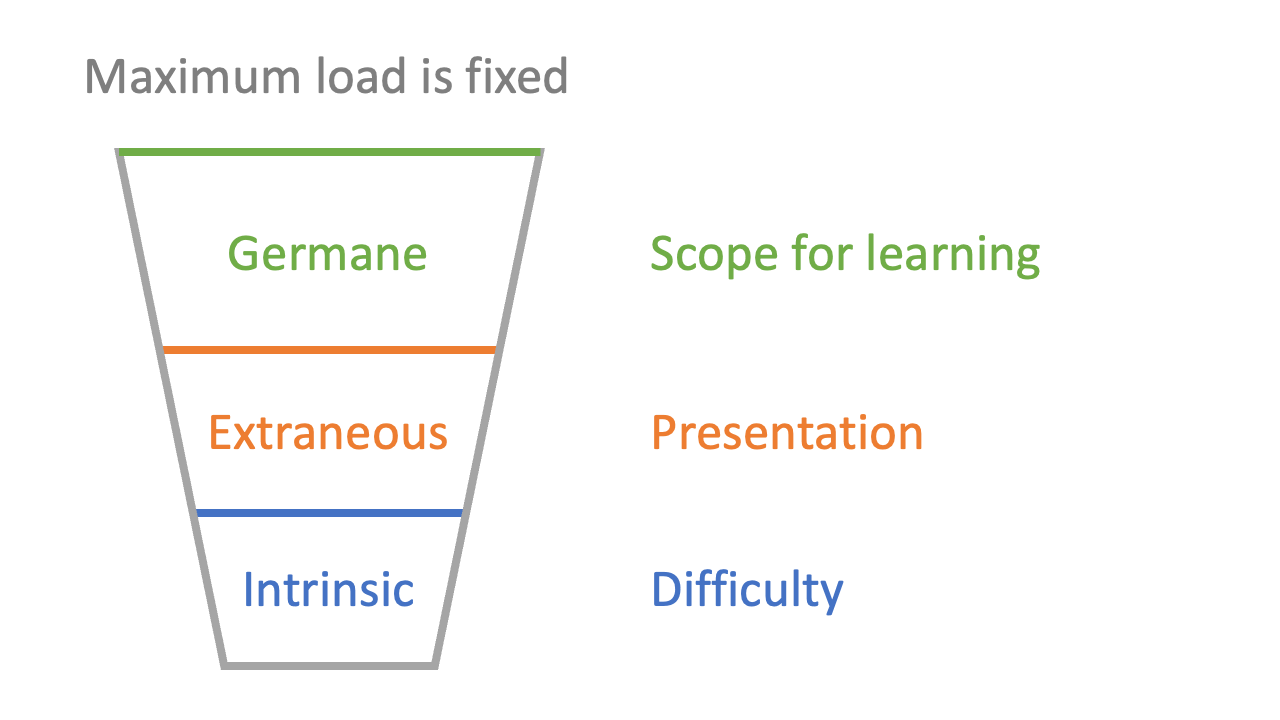
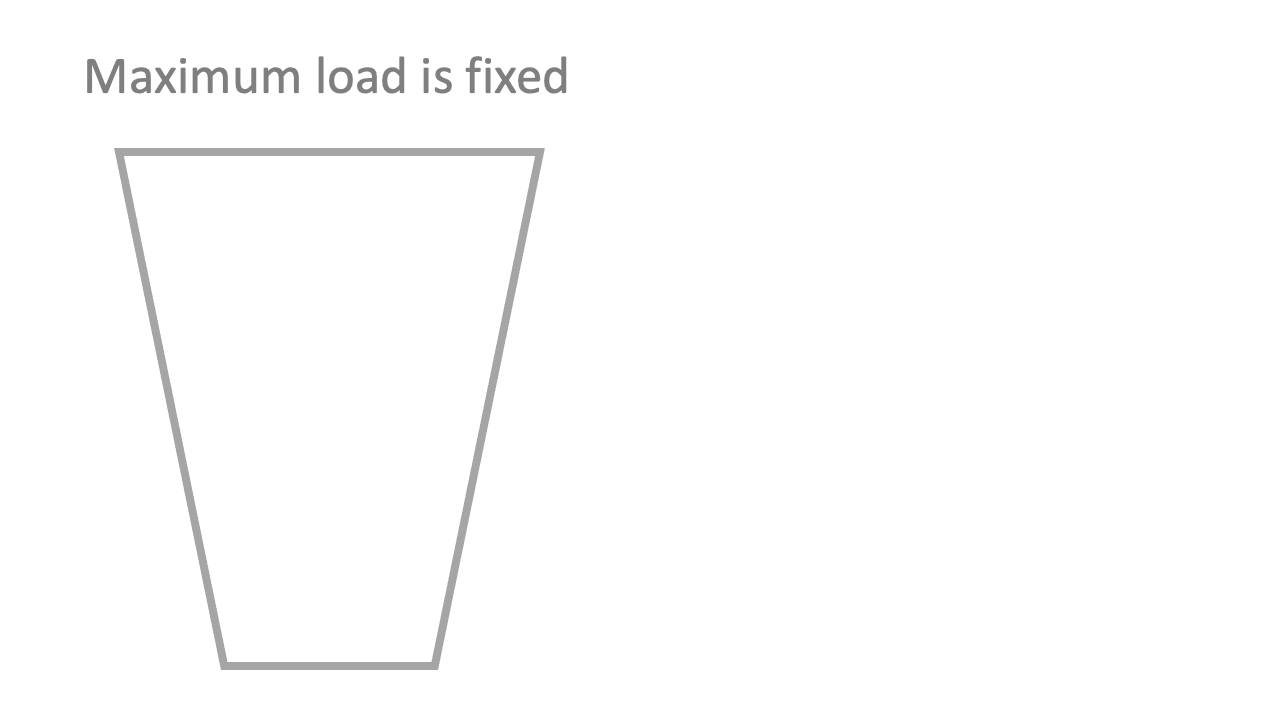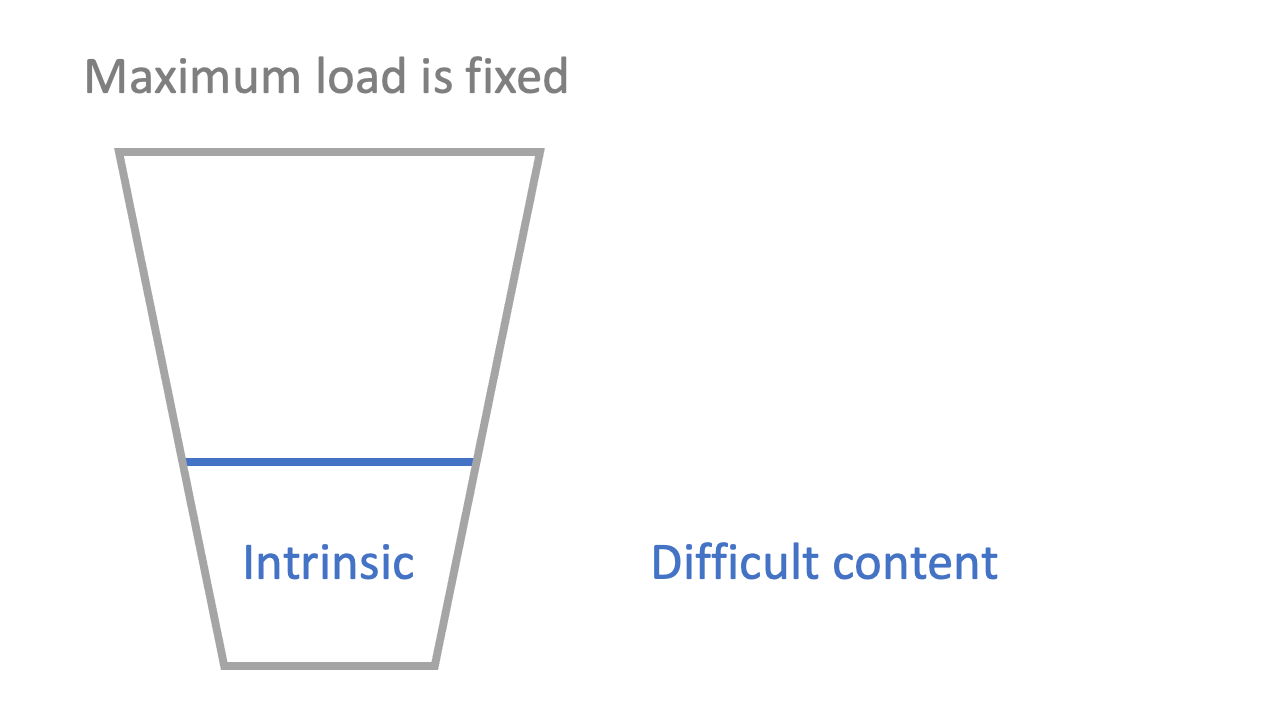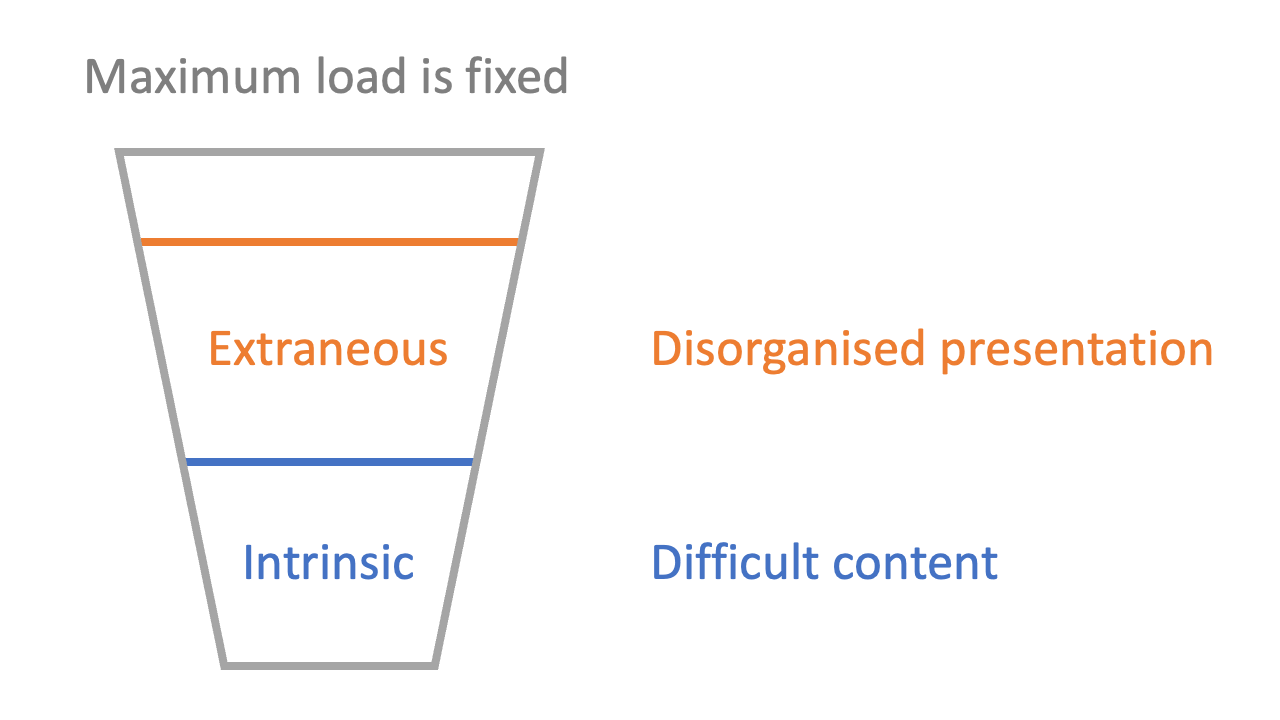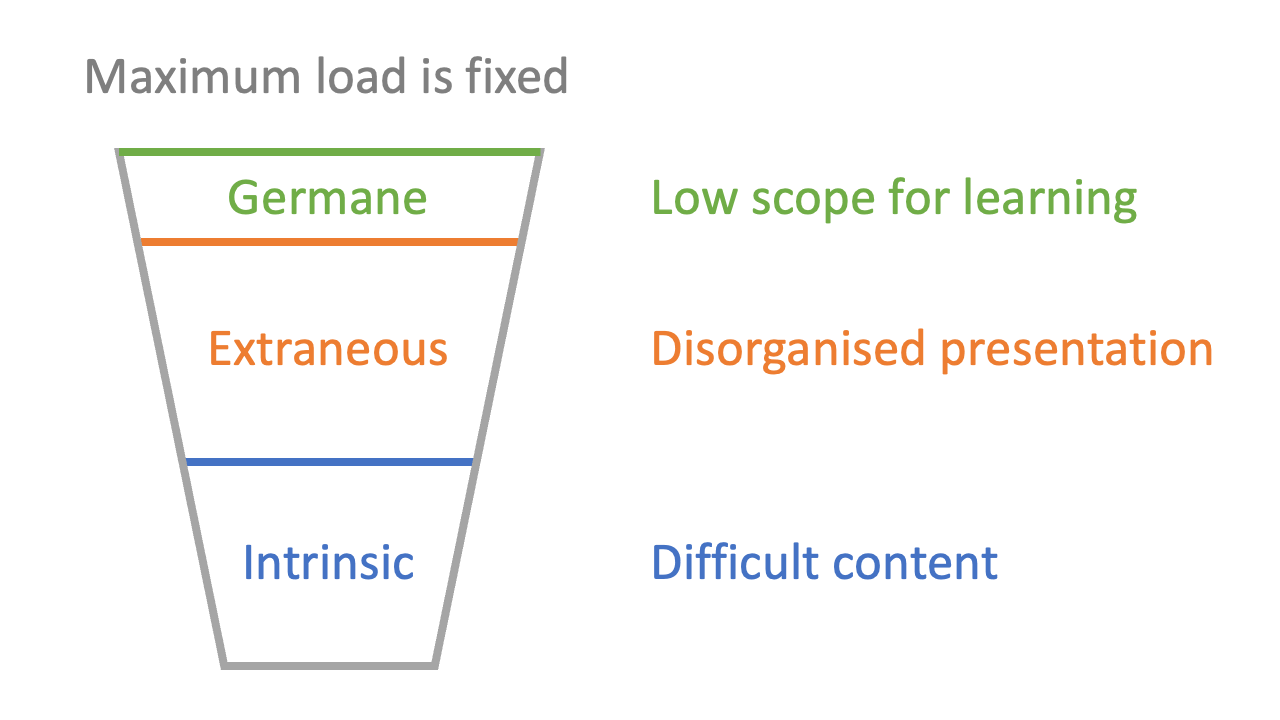
1: total working memory is limited
2: there is cognitive load associated with the difficulty of the information (‘intrinsic’ load)
3: there is cognitive load associated with the way material is presented (‘extraneous’ load)
4: there is cognitive load associated with internal cognition (‘germane’ load)
MON note: I had never encountered the word ‘germane’ before reading about Cognitive Load theory. It seems to mean ‘relevant’ or ‘related’.
The stickiest presentation of Sweller’s model I have seen is the ‘cup’ analogy. Just as a cup can only hold so much liquid, so a student’s head can only hold so much information in the short-term. Over-filling a cup is a dramatisation of what happens when information over-loads a student: they can’t hold everything they’re being given.
The Types of Cognitive Load
You can’t do too much about the intrinsic load as a teacher, except perhaps sequence the material logically and minimise content where possible. Some ideas are intrinsically hard, and some are intrinsically easy (though learners can reduce intrinsic load over time: see “Schema” below).
Extraneous load is about the processing of the presentation of information. If you have ever tried to read a book while a film is on in the background, you will probably have experienced high extraneous load. Similarly, a poorly-structured lecture or a confusing description of a scientific concept would increase the extraneous load of learning. Extraneous load should be minimised where possible, and doing so is a key part of effective teaching: good teachers present new information with low extraneous load.
Germane load is a good thing, and should be maximised: students should be exploring ideas, playing around with concepts, testing and refining their grasp of material. It’s not the case that germane load is the same thing as learning, but most of the deep engagement we desire learners to develop relies on exploiting germane load. For example, asking yourself questions like “why does that happen?” or “or this answer reasonable?” or “isn’t that reaction quite like the one silicon does?” is the kind of activity which exploits the surplus cognitive load “left over” when the intrinsic and extraneous loads have had their share. Good teaching is often about making space for germane load.
The Schema and the Chunk
A ‘schema’ is a mental model used to order and organise new information. The cognitive value of a schema is to compress a large amount of information into a more-digestible ‘chunk’ of knowledge. The ‘chunking’ of information in this way is the central mechanism for making efficient use of limited working memory. In Cognitive Load Theory, this can be expressed as reducing the intrinsic load of new information on working memory (by drawing on long-term memory).
The schema of “nucleophiles” might be one example: it’s a category which helps to describe a new molecule as an example of a familiar concept. If you know how nucleophiles behave, you will reduce the cognitive load of processing a nucleophile you’ve never encountered before.
Representational schemas are also important. If a student misses a crucial hydrogen on a skeletal structure in an E1CB problem, this might be theorised as a representational schema getting in the way of successful processing. The drawing and interpretation of skeletal structures becomes ‘second nature’ in time (it gets ‘chunked’), but until then students will routinely struggle with this form of representation.
This is a specific illustration of a useful general point: students have not yet fully mastered schemas, but their teachers have. What Cognitive Load Theory allows us to understand is that this means Chemistry is much harder for novices than experts. Experts can rely on schemas to compress the cognitive load of new facts into chunks, but novices can’t.
This suggests that teaching and learning can be radically different activities. Students are fighting for their lives in the Chemistry lecture! They are building schemas from scratch! Something you think is one concept might be three or four concepts to a beginner!
Chemistry as a high-load discipline
Chemistry as a discipline has a high cognitive load. The complexity of natural phenomena and the systematic appeal both to theoretical principles and raw data means that chemical scientists have a lot of information to process. Further, teaching through information-dense lectures and assessing through information-dense ‘problem solving’ exams are cultural features of the discipline which often serve to strain the working memory of even the most diligent students.
This means that cognitive load is a really useful theory for educators in Chemistry. Teaching this subject runs the very real risk of overwhelming the working memory of students who have not yet mastered schemas. Scholarly teaching addressing this issue deliberately can be highly effective; even simple changes can greatly improve the effectiveness of teaching.
Examples of Cognitive Load Theory in Chemistry Teaching
Example 1: scaffolding problem solving
Yuriev’s work on developing students’ problem solving skills might be framed as an attempt to develop scaffolding appropriate to the difficulties of high cognitive load in PhysChem problems. By mapping out the workflow of solving a problem, educators can help students to focus on the individual steps in a more focused way.
This can be seen as a way of controlling extraneous load: by constraining the sequence in which students engage with material, it is possible to emphasise the salience of particular information in each stage of solving a problem (“ok, now the flowchart says I need to ask myself whether this answer is sensible”). Such scaffolding is particularly useful when learners are new to a topic, and are building the absolute fundamentals. Once the cognitive load is compressed by chunking, the extent of scaffolding can be reduced. The scaffolding is designed to help develop their own schemas more rapidly than traditional instruction.
Example 2: programmed textbooks
The most famous ‘programmed’ textbook is The Chemistry of the Carbonyl Group by Stuart Warren. The format of the book is unusual. It teaches the reader a new thing about carbonyls (alphahaloketones readily tautomerise like this), then immediately gives them a problem which demands that they apply it (what would this 1,3-dicarbonyl do in base?). The reader tries it, and then turns the page, where they are presented with immediate feedback. The sequence of the book forces readers to build up their understanding of the topic and solve progressively-harder problems.
This ‘programmed’ structure might be seen as a way to navigate the high cognitive load of carbonyl chemistry. The extraneous load is minimised by the carefully-judged presentation of small pieces of new information, and germane load is arguably maximised through the provocation of immediate feedback. A student masters each step, and rapidly develops a really sophisticated grasp of solving carbonyl mechanism problems.
Example 3: gender in tutorials
My MChem student, Hannah Bruce, argued that gender was an important dimension in chemistry tutorials though an extraneous load analysis in Chapter 4 of her thesis. Interview work suggested that social elements of the experience could be described as extraneous cognitive load for women. It takes cognitive load to consider whether you should take up everyone’s time by asking a question, for example. Any increase in extraneous load comes at the cost of germane load, altering the educational value of a tutorial on the basis of gender. The presentation of any scientific information must relate to the social context it is delivered in.
Example 4: Dyslexia
If cognitive load is a good model for analysing chemistry teaching, then it’s worth reflecting on how certain disabilities might be particularly poorly-served by teaching which overloads students.
Dyslexia is typically characterised as a condition in which phonological (very loosely: language) processing is limited. This is often recognised when students need to construct phonological objects (e.g. when they do an exam, they might be given ‘extra’ time), but is perhaps less well-recognised when students need to learn things. Do you expect students to read a chapter from a textbook? To read a PowerPoint slide before you flip to the next one? Follow detailed guidance on a coursework submission? Proof-read an email? All of these tasks may take longer for students with dyslexia than you might have thought. Chemistry already has high contact hours and high cognitive load; it’s interesting to consider whether this combination might be systematically exclusionary for students with dyslexia.
Example 5: Exams
Chemistry exams are typically described as having a high level of problem solving. This ‘high level’ normally means that the outcome of the problem is pre-defined, the method of solving the problem is familiar, and the specific details of the problem are unfamiliar.
So a first-year student might be asked to predict the pKa of an oxo-acid (pre-defined outcome), be expected to use Pauling’s rules (familiar method), and never have seen the molecule H3PO3 (unfamiliar specific details). If lots of students are getting the wrong answer, how can you change your teaching to change that? If students are mostly getting it right, are there ways of usefully asking more-challenging questions?
The extraneous load of the question will be reduced if students have built a schema which lets them chunk new information into low-load objects. This might relate to the specific language of the question, or the saliency of the number of P=O bonds in the formula. Perhaps in such a simple case, this might be expressed in the folk wisdom of “practice makes perfect”: one way to get good at Pauling problems is by doing lots of them. If you wanted to constructively align your teaching with this objective you might (for example) work through this kind of problem in a lecture.
Familiarity with the specific structure of H3PO3 is certainly helpful, too: students who construct H3PO3 as P(OH)3 will struggle to get the right answer, even using good reasoning. Similarly, mis-remembering Pauling’s rules (“was it 5 minus 8p?”) will give an incorrect answer through logical application of an incorrect rule. The way examiners award partial credit when marking will likely relate to how important they believe it is to recall such information. Sometimes - whether we intend to or not - we are simply testing memory rather than problem solving.
Conclusion
Cognitive Load Theory is such a useful tool, and it’s not too difficult to grasp. I think that makes it a peculiarly high-value piece of professional development for anyone teaching Chemistry.
I think its greatest value in this discipline is to emphasise how students need to work really hard to do things which feel easy to an expert. This kind of insight can make us a little kinder, and also make us all a little better at teaching.
Its second contribution is to say that students can become ‘overloaded’ if they are presented with too much information or poorly-presented information. Good teaching leaves cognitive space for students to play around with concepts.
In my experience, these insights often don’t sit well with the way teaching is discussed inside institutions. Teaching less material is often seen as “watering down” an education, in ways which perhaps privilege breadth of learning over depth of learning, and maybe the perspective of an expert over the challenges faced by learners. It’s good to think about this!
Further Reading
A 2006 paper by Johnstone and El-Banna on the link between working memory capacity and exam scores.
An extremely nice PowerPoint slide deck by Michael Seery, touching on key theoretical and practical considerations of specific cases of teaching. The YouTube recording of his “Labs as a Complex Learning Environment” talk is also excellent.
Crippen and Brooks usefully discuss the use of worked examples in Chemistry Education as a cognitively effective strategy.
An Education Chemistry article reflecting on Cognitive Load in the analysis of school pupil behaviours. Perhaps the most practical of these reading suggestions, tying the theory very closely to specific actions in the classroom.