New Textbook: Hypovalent Cluster Structures
New Textbook: Hypovalent Cluster Structures
I wrote a textbook on hypovalent cluster structures, which I had hoped to launch at the 2020 Dalton conference. The organisers made the correct move in cancelling the conference to restrict the spread of COVID-19, and so I thought I’d blog my way through my book launch presentation slides.
Before I get going with the presentation, please note that a .pdf of the book can be downloaded for free here for the next two years. Given that the book can only be ordered through Amazon – a company whose employment practices during this pandemic have been really troubling – I encourage you to download rather than buy. If you love it, get a physical copy when warehouse staff don’t have to risk their health and family to send it to you.
The rest of this blog will be the talk I would have given.
Introduction
Hello. I’m Michael O’Neill, an education-focused lecturer at the University of Oxford, where I’m the Associate Director for Assessment & Feedback in the Oxford Inorganic Chemistry for Future Manufacturing Centre for Doctoral Training.
I’m launching a short textbook today. It’s about hypovalent cluster structures, and it’s written in an unusual format. I’m here to argue why this kind of book is useful, and then tell you about how it’s been put together.
So, here we go.
Articulating the Problem
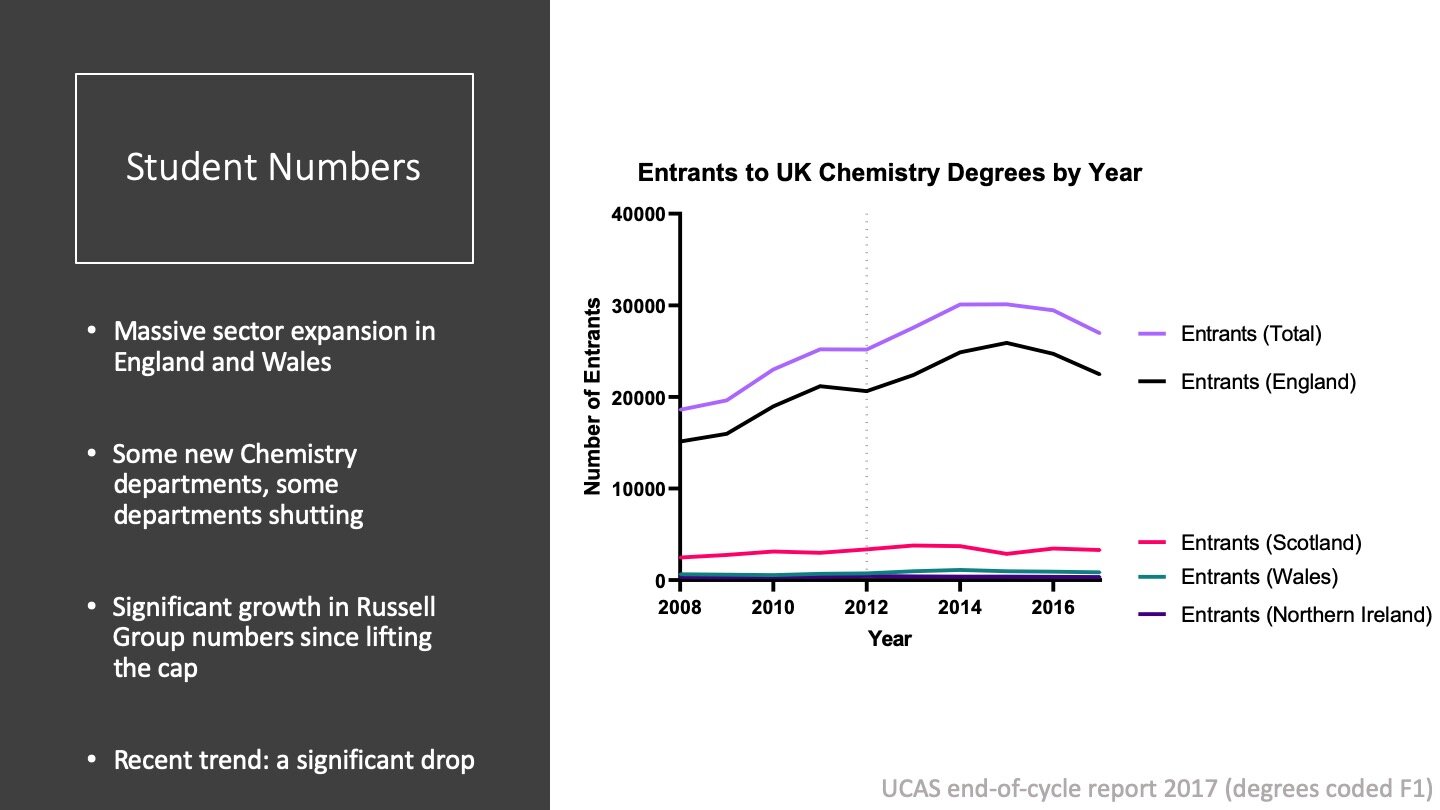
Undergraduate student numbers in UK Chemistry have fallen sharply the last couple of years, but have risen in the longer term. The graph shows student numbers in the nations by entry year from 2008 to 2017. UCAS data uses codes to group degrees, and these numbers include degrees which you might not think of as Chemistry. For example, Forensic Science is included with Chemistry in some coding schemes.
As you can see, the expansion in the sector has been driven by England, and the lifting of the government cap on student numbers in 2012 had a significant impact (dotted line). The growth has been driven largely by the growth of Russell Group Universities, but there is also some contribution through the creation of new departments.
The message I want you to take from this graph is that UK student numbers have increased when you look at the mid-term picture.
Student numbers are important to Universities economically. A HEPI report earlier this year calculated that UK Universities lose money on both domestic teaching and research. International student fees are much higher than home student fees, and this makes up most of the gap.
The absolute numbers in the HEPI report are quite shocking. When tallying research grants and REF income against the cost of research undertaken by Universities in the UK, there is a gap of over four billion pounds. This loss is mostly balanced using the fees of international students. Lots of the wonderful science being presented at Dalton would not have been possible without this money.
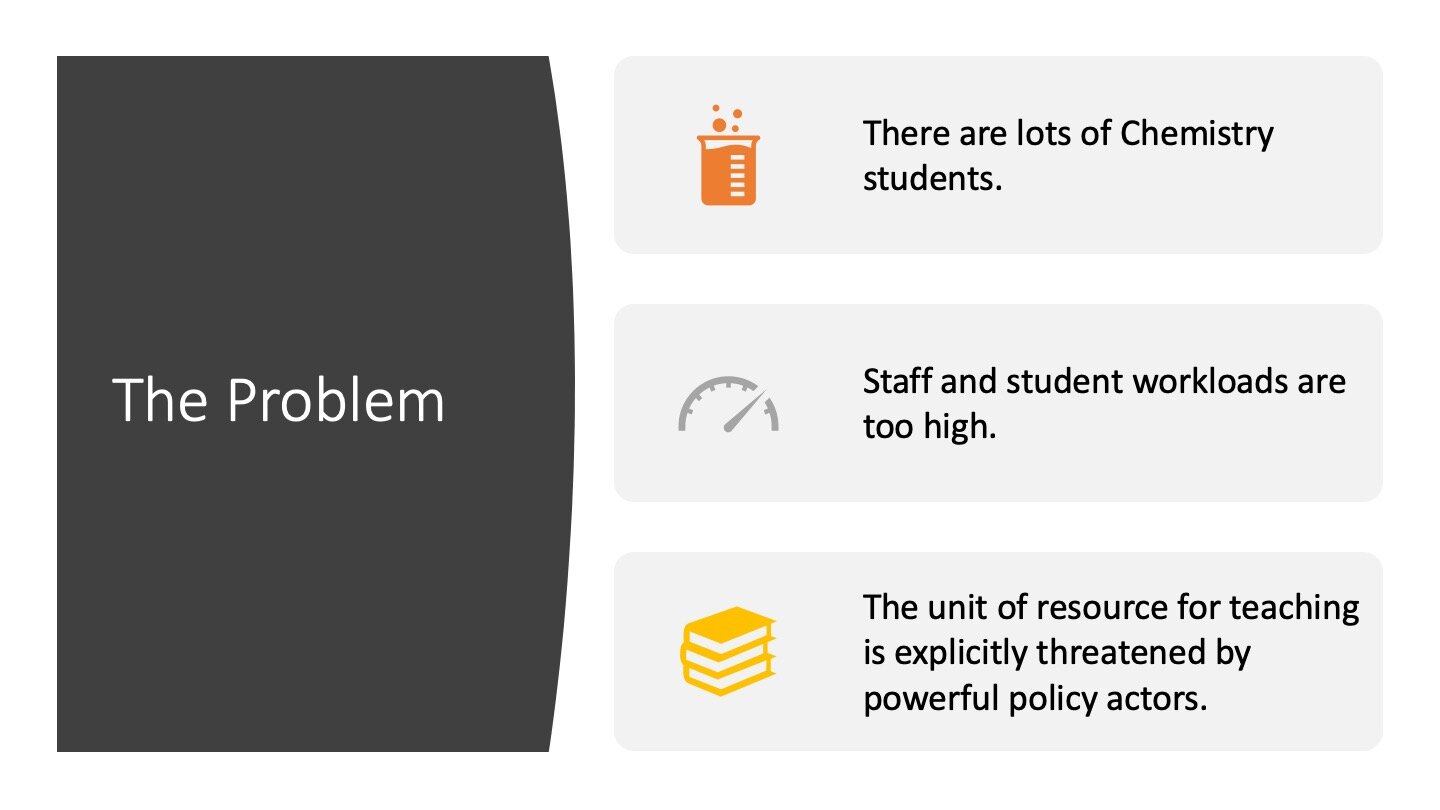
The Augar fees review looks unlikely to be implemented now, but it recommended a reduced home student fee. At the £7.5k/year level proposed, even teaching would have become financially unsustainable. I think this shows which way the wind is blowing with the policy agenda: the unit of resource available for teaching is politically threatened, partly because the way teaching subsidises research seems unfair.
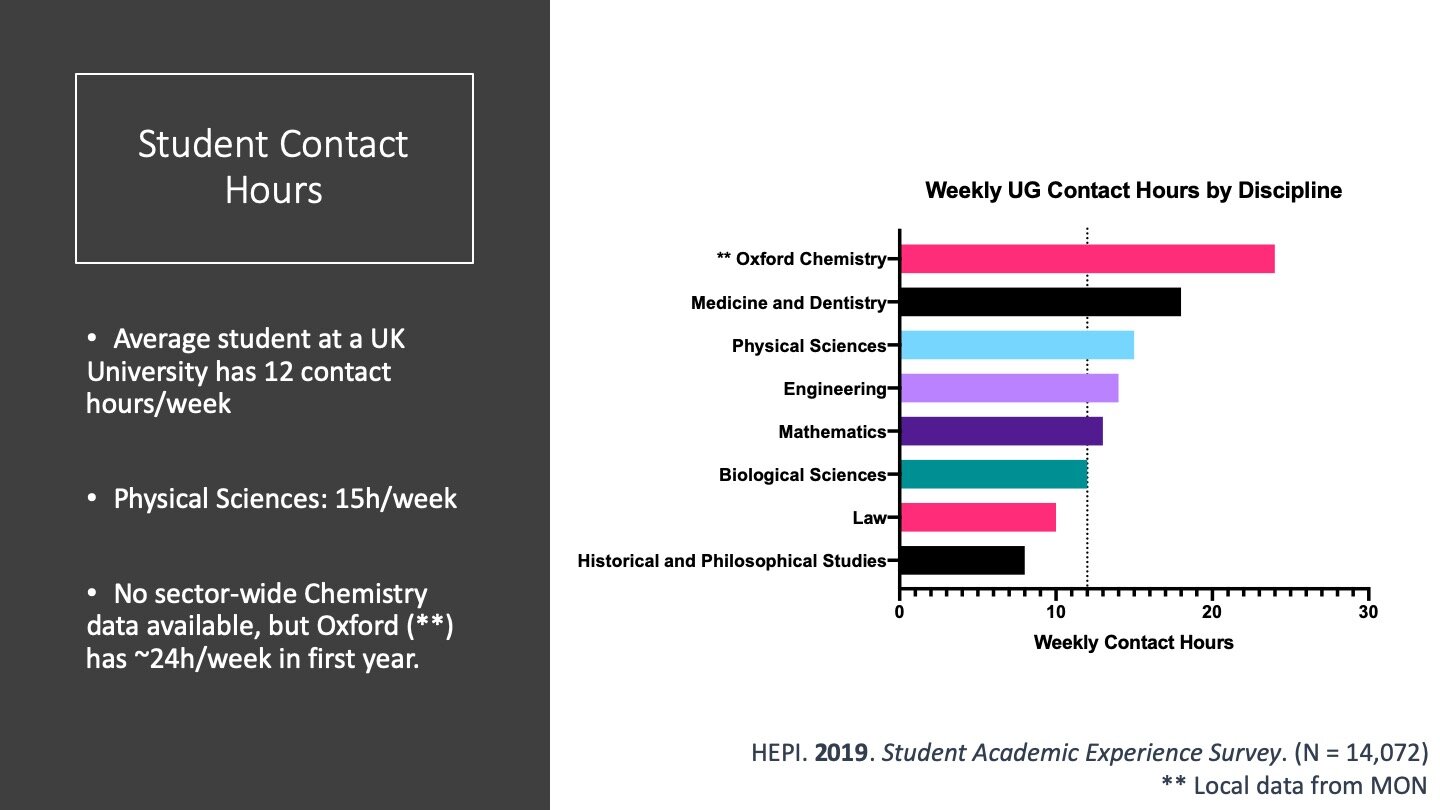
This policy threat is really important for Chemistry because we teach too much. The average undergraduate student in the UK reports having 12 contact hours per week, which I’ve plotted as the dashed line. The number for Physical Sciences students is higher at 15 contact hours per week. I couldn’t find national data for Chemistry specifically, but looked at Oxford’s first year timetable the week I put this data together. They did ten hours of lectures, twelve of labs, and two hour-long tutorials.
Perhaps that week was unusually busy for my first years, or perhaps Oxford is unrepresentative, or perhaps students in the sector-wide survey under-reported their contact hours. 24 is twice as big as the sector average of 12, though. 24 hours per week is more than engineering, more than medicine, and much more than law (and these degrees all grant graduates licence to practice in those professions, which a Chemistry degree doesn’t). We are massively over-teaching our students.
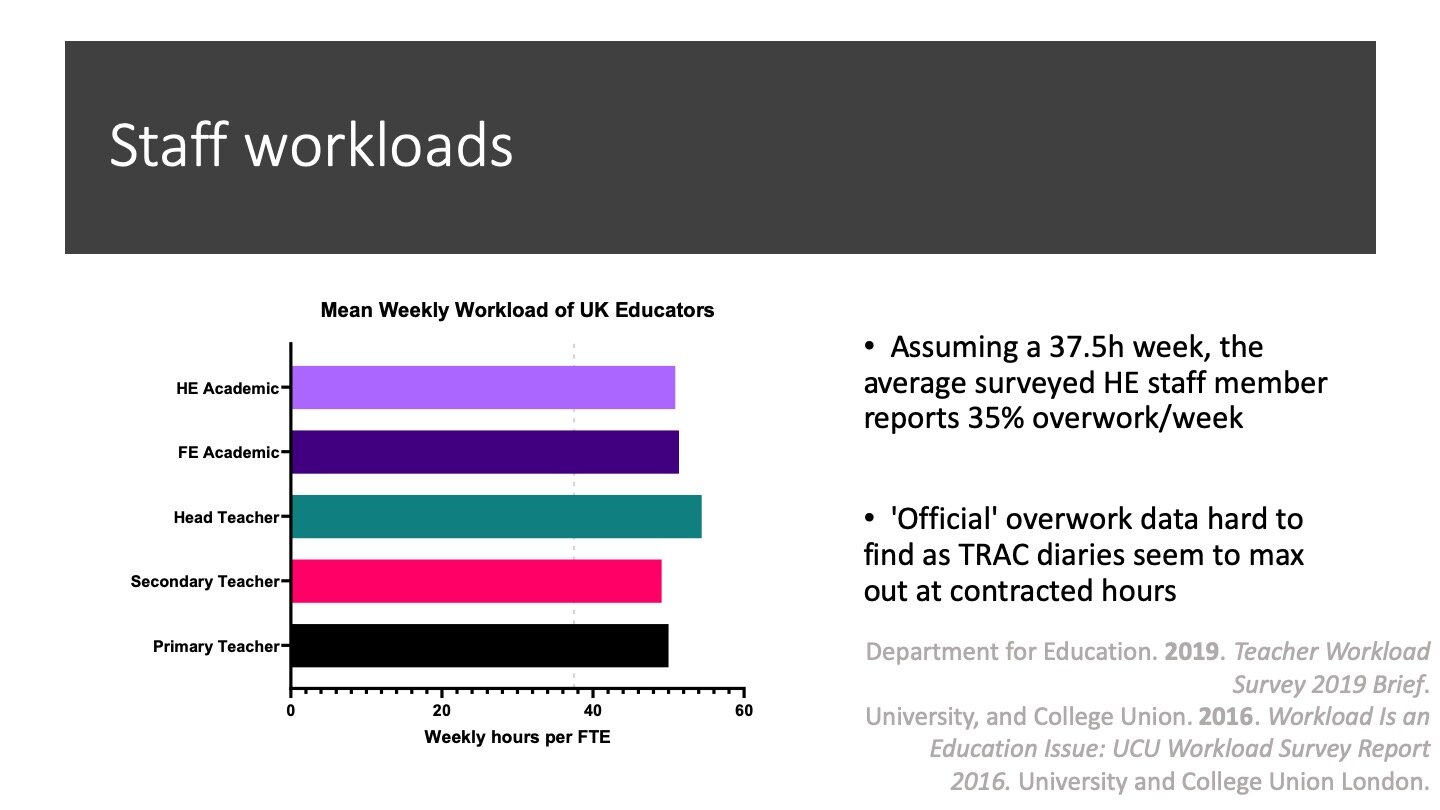
So, Chemistry has seen a growth in student numbers and teaches those students too much. I find this worrying for the students (and therefore for the inclusivity of our degrees), but it is also worrying for staff. Here I have found data for the average weekly workloads for various educators, and plotted the typical 37.5 hour week contract as a dashed line.
School teachers and FE teachers have their workloads measured by the Department for Education. The HE Academic data comes from a different source: a Universities and Colleges Union (UCU) survey in 2016. It is only fair to flag up that this is an organisation with a vested interest in over-reporting workloads, but I was surprised at how difficult it was to find non-partisan data. I may be mistaken, but I think the TRAC reporting approach to academic workloads makes ‘official’ sources such as HESA unreliable.
The broad point I want to make here is that academic staff are working too much. The average Higher Educator is working fifty hours a week by this data. For every slacker who’s only doing forty, there’s one doing sixty. In my view, these numbers are too high.
Statement of the Problem
So. There are lots of students, who are working too hard. Their teachers are working too hard as well. And domestic funding is both currently inadequate and also actively threatened by the policy agenda.
This constellation of facts means that Chemistry is in real trouble; in my view, our undergraduate teaching model is unsustainable.
Articulating a Solution
One solution to this problem is books. Books don’t take up contact time, scale efficiently with student numbers, and can reduce staff teaching load.
You might have used Warren or Vincent in your own undergraduate learning. They are written in a ‘programmed’ style which makes them very easy to learn from. Last year, I wrote a programmed book on assigning NMR spectra of main group molecules. This talk is about my new book on using Wade’s Rules.
The style of programmed books is very distinctive: they tell you something, then ask you to use that new piece of knowledge straight away. You write your answer to the question down, and then the book tells you how you did.
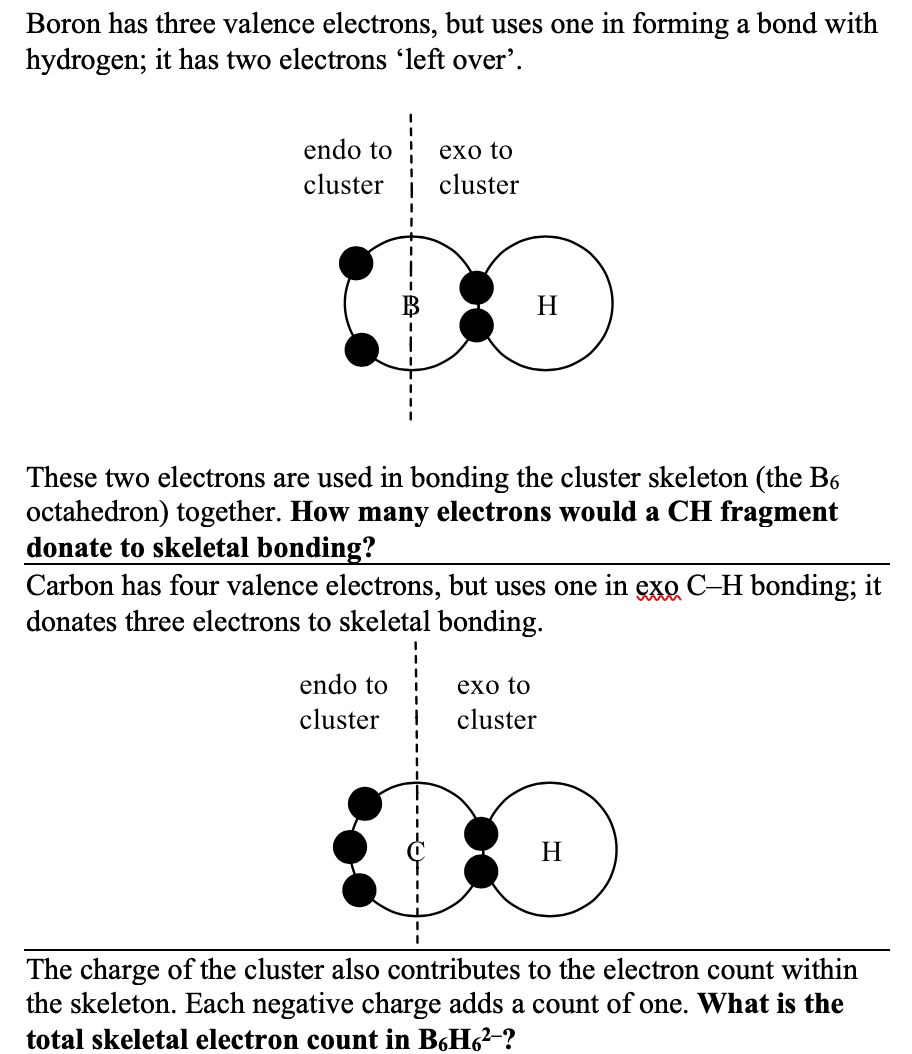
Concrete Example of Programmed Writing
Below is an excerpt from early in the book - sorry for the spellcheck squiggles! The reader is taught how to do the electron count for BH fragments, then challenged (all reader tasks are in bold) to do the same for CH. The answer is then given so the reader can check their understanding.
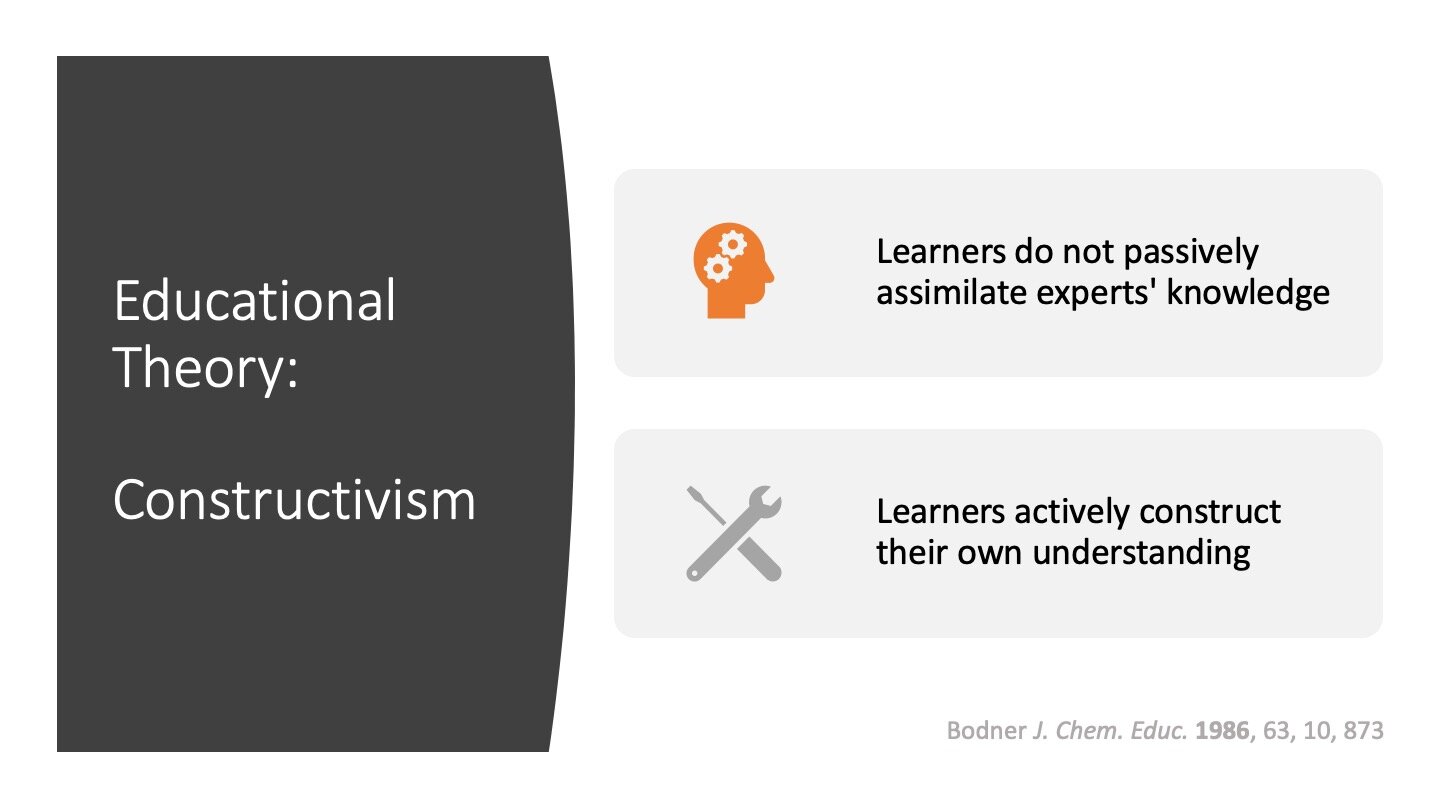
Constructivism
The reason the programmed style is so educationally powerful is that it embraces the idea that learners have to build their own knowledge. Lectures – perhaps including this lecture I’m giving you right now – are a terrible format for teaching if they are delivered intending to transmit knowledge. Lectures can be really successful when they recognise that the audience needs to build the ideas you already have for themselves.
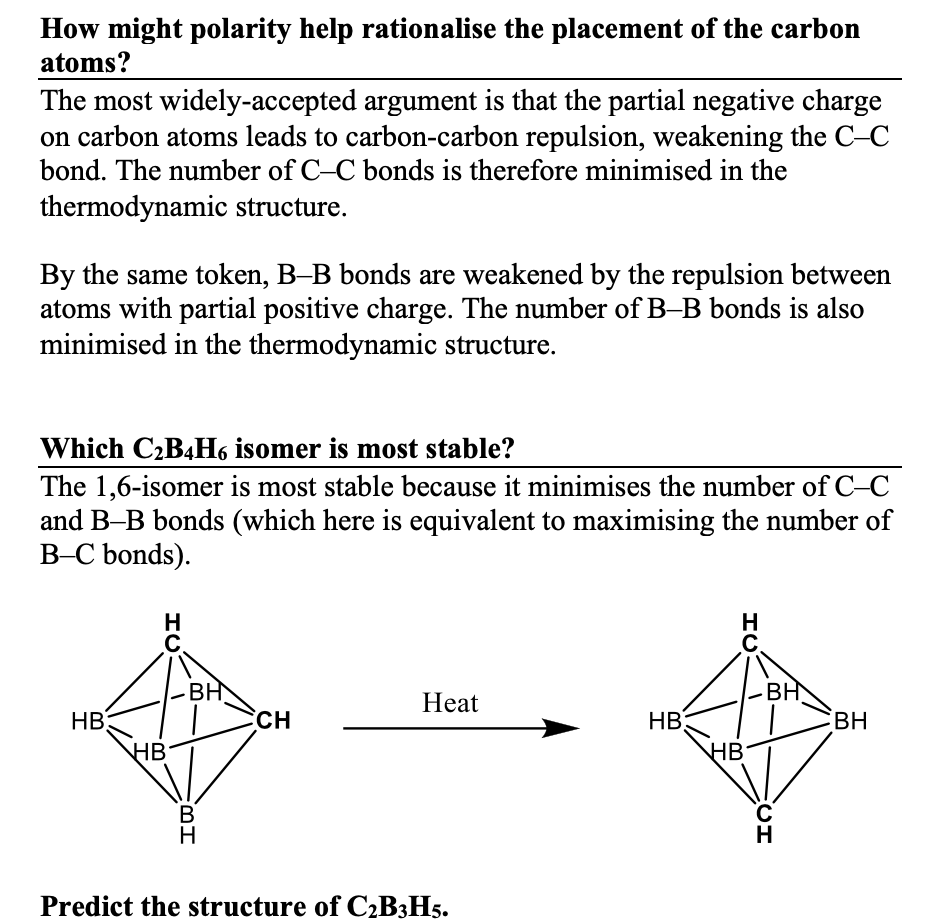
This vision of learners constructing their own knowledge is woven through the format of a programmed textbook. For example, in the excerpt below the reader is taught the energetic principle needed to determine the thermodynamic structure of a carborane and then challenged to use it to solve a similar problem. After they’ve had feedback on that problem, they’re challenged to try a similar (but less-structured) problem to integrate their new learning with their existing understanding of Wade’s Rules.
Cognitive Load Theory
This sequence of small learning steps is a response to the biggest problem in Chemistry Education: Cognitive Load.
Cognitive Load Theory is based around the idea that you can only process so much information at once. You may remember how hard it was when you started learning how to drive or how to write or how to use a Schlenk line: when everything is new, you’re bad at doing things.
Cognitive load explains this by suggesting that your capacity to process information is overwhelmed by the amount of new information. Once you become familiar with the new things, you don’t need to process so much information.
You get better at driving when you ‘just know’ where the clutch is. You get better at writing when you can draw the curly-cuh character without having to recall what it looks like. You get better at using a Schlenk line when you internalise that an up tap is nitrogen and a down tap is vacuum.
Chemistry has a huge cognitive load. Our subject is vast. We cram the edges of biology into this degree. We stuff some physics in. We fill it up with materials and pharmaceuticals and calculus and modelling and scale-up and analysis. And then we do labs!
But beyond sheer content coverage, the complexity of the ideas we use is really high. Chemistry is a really tough degree to study; this makes it a difficult degree to teach.
We have double the contact hours of the average undergraduate student, and we have a discipline whose defining characteristic is its breadth and whose academic rigour emphasises depth. Students can learn about Taxol at nine and ceramics at ten and selection rules at eleven. They have so much new stuff to deal with; the cognitive load they experience is huge.
Our core challenge when teaching undergraduate is therefore to help students to process the information which lets them build their own knowledge. The small steps in a programmed textbook help students to gather the conceptual building blocks they need. The frequent questions force students to construct their own knowledge using those blocks. The immediate feedback helps them to construct that knowledge correctly.
A programmed textbook could replace lectures. Maybe not a whole lecture course, but perhaps you could use this book instead of five or six of those lectures. Such replacement reduces student contact time, reduces staff workload, and reduces the pressure on resources like lecture theatres. It’s a huge win.
Perhaps you would want to spend that time doing something else; you could run an extra workshop for students if you wanted. Or perhaps you just want to save that time; staff and students are already working way too much.
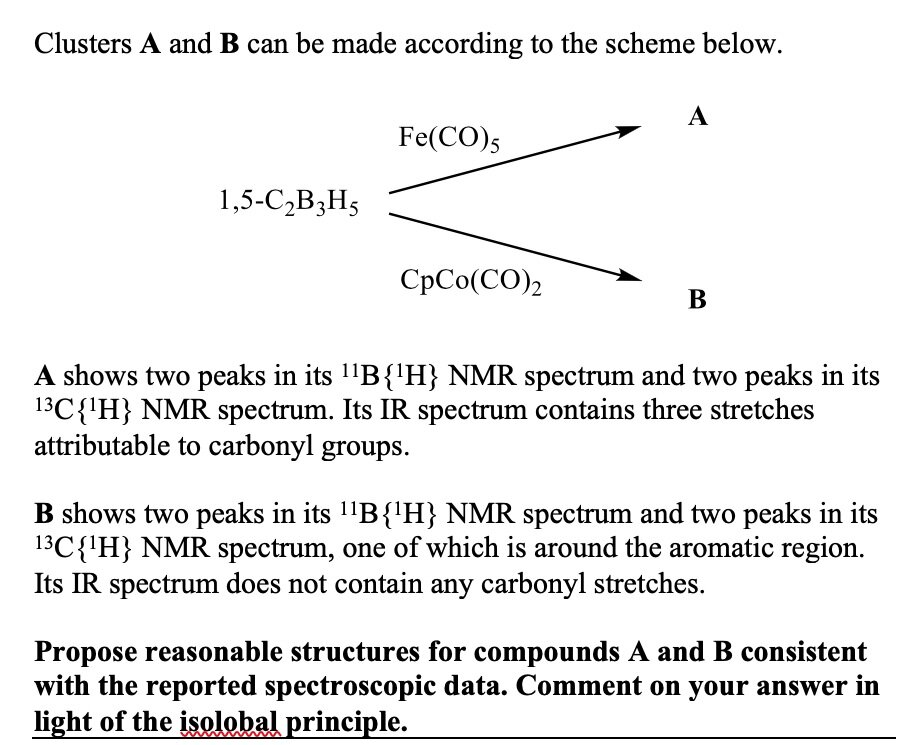
Below is an excerpt from the last chapter in the book. The complexity of the problem has reached a really respectable level, integrating metallofragment counts, isomers, and analytical data. A student working through this book should be able to solve this sort of problem by the time they finish. You will judge for yourselves whether this sort of end point could be useful to your own students.
Publishing Model
Just to close, a quick note on my publishing model. I am self-publishing using Amazon’s Print On Demand service (but have had really generous reviews from students and academics through my editing process). This is a weird way to publish, and is quite interesting.
If you order a Print on Demand book, it will be printed for you. It isn’t waiting on a shelf for some one-day buyer: it doesn’t exist yet. This means that Amazon doesn’t have to hold any stock for me, so they reduce their warehousing costs.
This makes edits really easy. I can upload a new file and update the text within an hour. This allows me to improve the book as I go; if you ever find any ways of improving the text, please let me know.
Self-publishing means I can control pricing. So long as Amazon doesn’t lose the cost of printing the book (which includes some profit margin), I can pick the price. I have set this to £4.99 in the UK, and similarly round numbers in other markets. Many of our students are really poor; I hope that £5 doesn’t bar them from Wade’s Rules.
Thank you for your time, and thanks again to the organisers for my chance to present an Education talk to you. If you have any questions, I’d love to hear them.